Artificial Photosynthesis
Plant photosynthesis converts solar energy into chemical energy in the form of sugars, cellulose, lignin, etc using CO2 and water as the raw materials. It is regarded as the most important chemical reaction on the Earth, which has inspired the development of artificial versions of photosynthesis, i.e. (1) the splitting of water into hydrogen and oxygen, and (2) the conversion of carbon dioxide into organics via sunlight. An important challenge in artificial photosynthesis is the development of catalysts that should be sufficiently efficient, stable, inexpensive, and capable of harvesting the abundant visible light in solar spectrum. There are countless trials to establish stable systems for this purpose, mostly based on inorganic semiconductors with appropriately engineered band-gap and noble metals to promote the “extraction” of electrons. These materials include metal oxides, (oxy)sulfides, and (oxy)nitrides. In our group we are investigating polymeric and organic-inorganic hybrid materials with controlled nanostructures as potential energy transducers for artificial photosynthesis for such applications as water splitting, CO2 reduction, environmental purification, and organic synthesis.
MOFs Co-catalysis
MOFs as redutive co-catalysts for CO2 photoreduction
Metal–organic frameworks (MOFs) have shown great promise for CO2 capture and storage. However, the operation of chemical redox functions of framework substances and organic CO2-trapping entities which are spatially linked together to catalyze CO2 conversion has had much less attention. Reported herein is a cobalt-containing zeolitic imidazolate framework (Co-ZIF-9) which serves as a robust MOF cocatalyst to reduce CO2 by cooperating with a ruthenium-based photosensitizer. The catalytic turnover number of Co-ZIF-9 was about 450 within 2.5 hours under mild reaction conditions, while still keeping its original reactivity during prolonged operation.
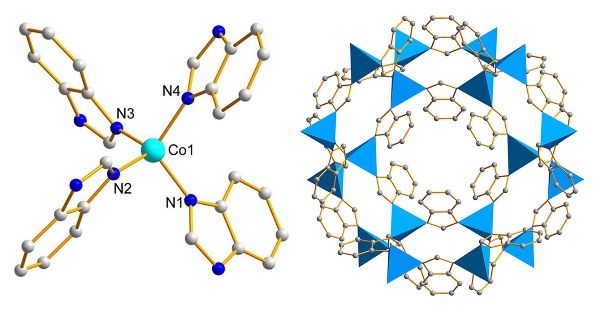
Scheme 1. Chemical structure of Co-ZIF-9. (left) Ball-and-stick representation of the second building units showing the coordination environment around Co. (right) Packing diagram of Co-ZIF-9. Hydrogen atoms are omitted for clarity. Color code: Co (light blue), C (gray), N (blue).
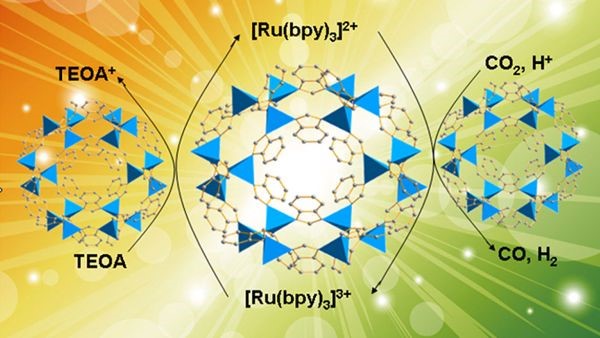
Figure 1. A cobalt-containing zeolitic imidazolate framework (Co-ZIF-9) has been used as a stable metal–organic framework cocatalyst with a photosensitizer to reduce CO2. It combines benefits of the nanoporous characteristic of Co-ZIF-9 for CO2 capture/activation and the catalytic redox function of cobalt centers. bpy=2,2’-bipyridine, TEOA=triethanolamine.

